Top 10 The Most Astounding Atomic Facts
10. There are around 500,000 atoms in one human hair.
The expression “a hair’s breadth” refers to the width of a human hair and is often used to describe an absurdly little object. Considering how thin hair is, it’s a fair analogy. In comparison to other objects, such as a rope or your arm, hair is rather narrow. As compared to atomic units? Very little.
On average, there are 500,000 atoms in a human hair. In 2004, scientists created the first microscope with a 0.6 angstrom resolution, which allowed them to see objects well. Your hair is 500,000 times larger than an Angstrom, the shortest wavelength of light. The reason behind this is that the typical atomic diameter is one angstrom. The number of atoms per unit of hair can range from 300,000 to 1 million.
9. The human body has more atoms than the universe’s stars.
Imagine the complexity of the human body when you consider that just one hair requires over 500,000 atoms to traverse it. In a nutshell: yes. With more atoms than stars in the universe, the human body is dense. Behold you, transcending the boundaries of space and time. Very respectable.
Carbon, oxygen, hydrogen, and nitrogen make up the majority of your atoms, which is ironic. That’s almost all of you; the other 6% consists of minerals like gold, copper, magnesium, calcium, and a host of others. What does that imply, though, when expressed in terms of absolute numbers? Is the number of stars in the cosmos known?
Since our limited view of the cosmos prevents us from knowing for sure, we can only conjecture on the matter. But based on what we have observed, we can calculate a ballpark. We have arrived at a figure of approximately 200 billion trillion. You might as well simply write it down as 200 squidillion; your mind can’t make sense of it any other way than as “a lot.”
The number of atoms in the human body can be approximated as 10^27. It’s one octillion. Some estimates put your number of octillions at 6.5. For the simple reason that atoms are constantly moving through your body (in and out of your lungs, your digestive tract, and your excretory system), it stands to reason that we all possess a small fraction of the atoms that have ever been in every single human being and a single atom from every single breath anyone has ever taken. Very well done.
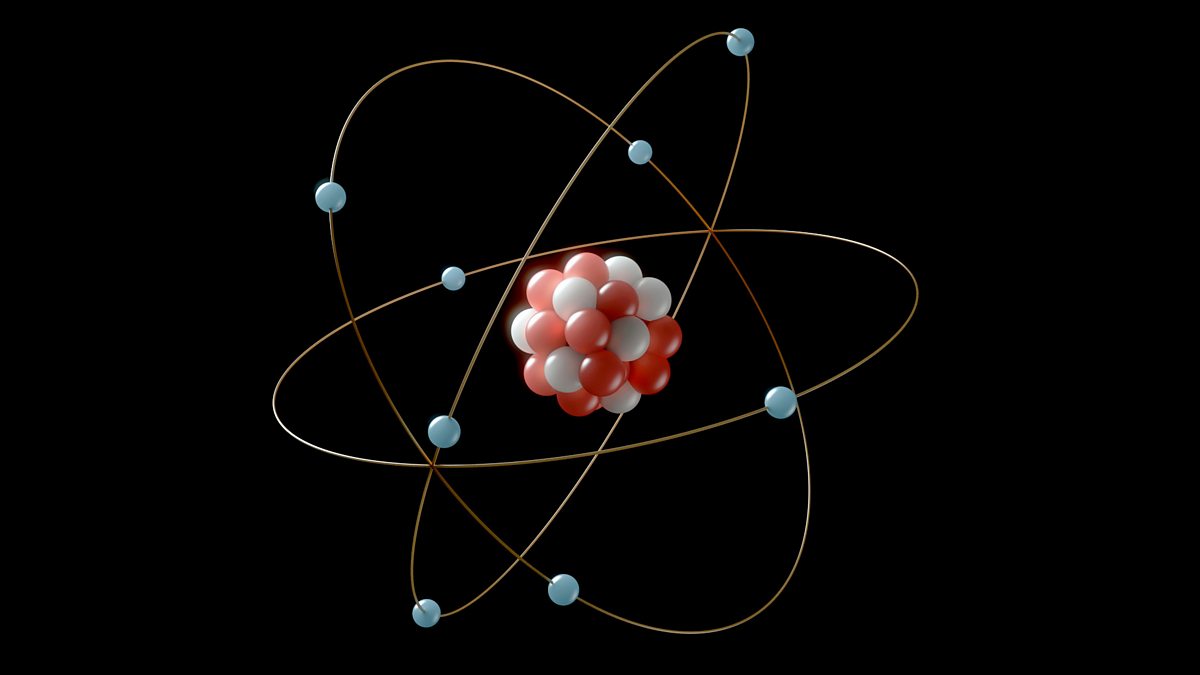
8. A nucleus is thousands of times smaller than an atom.
Most of us have a hard time relating to or making sense of atoms in our daily lives due to their minuscule size. Certainly, they do exist, but while you’re occupied with preparing dinner or attempting to pick up after the dog, does it really matter? Very little.
Nonetheless, the outcome might be truly remarkable when you have the opportunity to view things in context. For example, electrons circle around the nucleus of every atom, which contains the proton and neutron.
Of course, the nucleus is smaller than an atom, but by how much? The nucleus would be easily portable if an atom were the size of a stadium. If you require further details, two comparisons using various stadiums could be useful. Like football? Atoms with nuclei the size of blueberries have the mass of a football stadium. Notoriety in sports? You are now staring at the nucleus of a ping pong ball.
An atom is around 100,000 times larger than its own nucleus, just in terms of the basic numerical difference.
7. William Shakespeare and You May Have Up to Two Hundred Billion Atoms in Common
So, let’s take a closer look at what we said earlier: that you share atoms with every single person who has ever lived. In these conjectures on the possibility of sharing atoms with the bard, Shakespeare is the common figure. Sure, you undoubtedly breathed in Shakespeare’s air, but hydrogen makes up the bulk of you and oxygen comes in as a close second. On the other hand, he may have once carried your carbon.
The carbon atom in Shakespeare’s sandwich or chicken had a remarkable journey from being formed in a star, sent hurtling through space in a comet, dispersed across the planet, and then passed through various possible life forms for millions of years. It is also entirely possible that it came to you from him over time.
Looking behind the carbon, some scientists have hypothesized that you shared billions of atoms with Shakespeare, not just a handful. Perhaps up to 200 billion. Everything from the air he breathed to the water you drank and the food you ate was impacted by his perspiration. Of course, Shakespeare is only one example. It doesn’t matter who you choose—any human being in history will do.
6.Your Atoms Are Exchanged Annually (98%)
As a person composed of octillions of atoms, you are incredibly active on an atomic level, but there is even more going on than meets the eye. Your atoms are in a perpetual state of motion, never remaining still. everything’s all of everything, not just the air you inhale.
New cells are always being produced by your body. The skin regenerates as you shed dead cells. Your hair regrows. You perspire, you defecate, and you expel waste. You are constantly constructing and dismantling your identity. No matter how old you are, you are always generating new blood, bones, and everything else. Every year, you replenish your body with new atoms—98% to be exact. Your bones and even half of your carbon atoms are replenished every few months.
What binds us all to people like Shakespeare on an atomic level is this cycle of destruction and reconstruction. If your molecular weight is six octillion and your annual rate of atomic replacement is ninety-eight percent, then you, like everyone else, will lose 5.88 octillion atoms each year. This means that each year, you must consume 5.88 octillion more calories, some of which will naturally come from other sources.
5. Exhaling Carbon Atoms Causes Weight Loss

There is a lot of focus on the weight loss industry, but not enough on the science behind what it means to lose weight. A lot of people talk about how important it is to burn calories through activity and a healthy diet, but what does it mean in practice? What happens to the mass that you lose when you exercise if you want to reduce weight? It is necessary to physically remove atoms—a large number of them—from your body.
You can see the results of your weight loss via the holes in your face. Exercising causes the atomic bonds that comprise fat molecules to break down. However, at the atomic level, everything must have a destination. As you exhale carbon dioxide during exercise, a portion of the fat molecules are transformed to this gas. Your personal trainer was correct in reminding you to breathe correctly; it is the mechanism by which the fat is actually being expelled.
The three simple building blocks of life—hydrogen, oxygen, and carbon—make up triglycerides, the molecular building blocks of fat. Half of them are transformed into water, which can be eliminated by perspiration or urine, while the other half are turned into carbon dioxide.
If you lose 10 pounds, around 1.6 of those pounds will be water, which is easily reabsorbed when you drink enough of water. But you’ll have to eat or drink enough to replenish your fat stores if you want to regain the 8.4 pounds you’re likely to lose.
4. Our Galaxy Is Not the Origin of All Atoms in Your Body
The atoms that make up your body still have a ways to go. The world is what it is, and they might have been in every other person’s life at some point. Your atoms didn’t originate from this planet; the cosmos is enormous.
Every one of your atoms isn’t native to our planet, our solar system, or even our galaxy. Our Milky Way is home to atoms from other galaxies, accounting for around half of our total mass. No Captain in Starfleet history has ever ventured farther into space than you.
As supernovae blast off massive quantities of matter into space, those atoms are drawn in by nearby galaxies. A portion of that found its way to our solar system, our planet, and ultimately to you.
3. Graphene’s Extreme Versatility and Atom Thickness
Since we have been concentrating on atomic numbers up to this point, why not take a step back and admire graphene? The word has been popular in science news for at least ten years, so you could be familiar with it. Articles abound discussing graphene’s potential uses, but how is this relevant to the topic of atoms?
Graphene has the ability to create objects with unprecedented levels of thinness. Something with a thickness of just one atom can be created using graphene. In 2008, scientists created a graphene balloon that could contain gases despite its extremely thin thickness (only one atom). Attempting to visualize this while being aware of the number of atoms in a human hair and its strength is nearly inconceivable.
Graphene sheets that are only one atom thick have been hailed as the next great supermaterial. Supermaterials for computer chips, construction materials, and more are on the horizon; they are stronger than diamond and more conductive than gold. Making and using graphene is a costly and time-consuming process, which is a major issue. On the other hand, we will witness the power of a single carbon atom when that day comes when it isn’t.
2. You’ve Never Heard of the Estimated Number of Atoms in the Universe
We’ve gone over some large numbers previously, so now let’s tackle the largest one. By drawing parallels between cosmic stars and human atoms, we arrived at the absurd octillion figure. Who cares about atoms in the vast cosmos however?
One of the joys of this math is how completely theoretical it is. We can undertake some elaborate extrapolations using our current understanding of the universe’s galaxy population and its constituent atoms to arrive at an answer that is so ludicrous that it’s nearly impossible to tell if we’re exaggerating or not without resorting to Google.
It has been suggested that there are between ten quadrillion vigintillion and one hundred thousand quadrillion vigintillion atoms, based on estimations of the known universe, which is not the entire universe. A vigintillion? What on earth is that? The range is 10^78 to 10^82 atoms, if you like numbers. A one with eighty-two zeroes, then. Who dared to call it by that name?
1. If we were to eliminate the empty space in atoms, the entire human race could be condensed into a sugar cube.
Imagine a man who is 6 feet 2 inches tall and weighs 200 pounds. He might consist of six octillion atoms, as far as we know. Even if there appears to be very little room for all those atoms, keep in mind that they are also completely empty. An atom contains a nucleus, as we mentioned before; nonetheless, the atom itself is 100,000 times larger than the nucleus. There is empty space between all the molecules that comprise matter as well as between each individual atom in a molecule. No matter how dense a specific particle may appear, matter is actually empty.
Each of the eight billion cells that make up the human body is utterly devoid of any substance whatsoever. If we could compress atoms to eliminate all the empty space, we could squeeze all of humanity into the area of a sugar cube. That’s how much empty space there is in your atoms.
Because it is extremely dense material, the sugar cube of humanity would be just as heavy as all of humanity, so it would be futile to attempt to lift it. Because matter is 99.9999999% empty, this is all possible. Compared to everything else, we’re specks, as thin as air and hardly perceptible.
SEE ALSO:
Top 10 Highly Valuable Patents Disclosed To The Public